02.12.2019
High-Performance Density Gradient Media for Purification of Leukocytes from Blood
Engineering & Biotechnology, 2019
An article in Genetic Engineering & Biotechnology written by Shanghao Li, PhD and Miodrag Micic, PhD, ScD, describes formulations that isolate mononuclear and polymorphonuclear leukocytes with high yield and viability. The article titled “High-Performance Density Gradient Media for Purification of Leukocytes from Blood” can be read in its entirety below.
Isolation of viable mononuclear and polymorphonuclear cells from blood serves as the starting point for a wide spectrum of immunology and cell biology studies. Unlike red blood cells and platelets, all white blood cells are nucleated and can be classified by their nuclei structure as mononuclear or polymorphonuclear cells.
Lymphocytes and monocytes are the two main categories of mononuclear white blood cells with one-lobed nuclei in the immune system to defend the body from infection, cancer, and other foreign invaders. In humans, lymphocytes make up most (70–90%) of the peripheral blood mononuclear cell (PBMC) population, followed by monocytes (10–30%), and a small percentage of dendritic cells (1–2%). Distinguished from mononuclear leukocytes, polymorphonuclear leukocytes have the varying shapes of the nucleus, which is usually lobed into three segments. Neutrophils are the most abundant polymorphonuclear leukocytes, and the other types (eosinophils, basophils, and mast cells) have much lower populations.
For many immunological and cell culture workflows, the first step is to specifically isolate leukocytes from the blood. Early methods for isolating leukocytes involved mixing blood with a compound that aggregates the erythrocytes and, to a much lesser extent, the leukocytes. Upon centrifugation, these aggregated erythrocytes precipitate due to their greater density and form a pellet, while the leukocytes remain in solution. However, the recovery of leukocytes using this method can be unsatisfactory. Cells can be trapped by and sedimented with the aggregated erythrocytes, resulting in broad spectrum of damages to the isolated cells.
In 1968, Bøyum introduced a more convenient and rapid separation using centrifugation through a Ficoll-sodium metrizoate solution.1 This separation method takes advantage of cell density differences of the components in whole blood that, when centrifuged in the presence of a density gradient media, exhibits a unique migration pattern through the medium allowing distinct cell populations to be fractionated. The whole process is performed at a lower gravitational force field without the introduction of aggregating reagents, thus preserving cell viability.
A pain point for many researchers is how to specifically isolate mononuclear and polymorphonuclear cells from blood with high yield and viability. MP Biomedicals offers three products for the isolation of mononuclear and polymorphonuclear cells from human peripheral blood as well as from bone marrow and umbilical cord blood. These isolation products allow users to obtain a cell fraction that may serve as a starting point for further separation of specific cells using cell sorting or similar techniques.
ICN Biomedicals, the predecessor of MP Biomedicals, was one of the first companies to introduce commercial ready-to-use kits for lymphocyte separations. Since early 1970s, the Lymphocyte Separation Medium (LSM™), LymphoSep®, and Mono-Poly® Resolving Medium have been used for many applications by researchers worldwide.
Mononuclear cell isolation for research use only
The optimized LSM produced by MP Biomedicals has a unique formulation where sodium diatrizoate is successfully substituted for sodium metrizoate. LSM allows for the separation of lymphocytes not only from human peripheral blood, but also from bone marrow as well as umbilical cord blood. It has a density of 1.077–1.080 g/mL consisting of 6.2 g Ficoll and 9.4 g sodium diatrizoate per 100 mL of solution.
LSM was designed to ensure maximum yield of mononuclear cells with more than 96% cell viability of lymphocytes in one-step centrifugation. (The separation principle used by LSM to isolate lymphocytes from the other blood constituents is summarized in Figure 1.) This time-tested and -proven separation medium has been cited in over 2200 scientific publications.
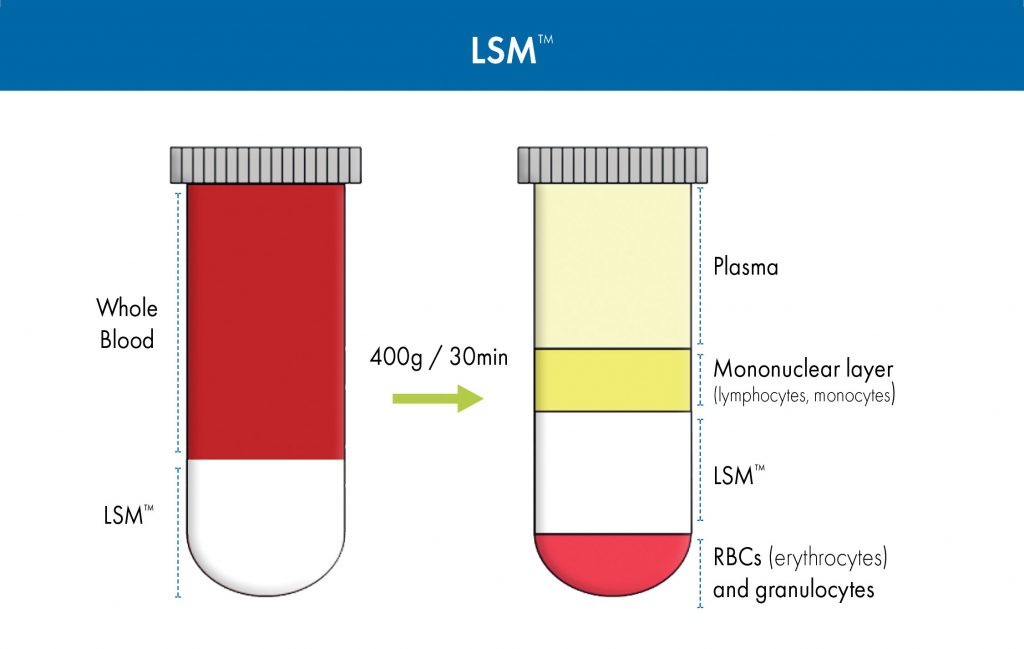
Figure 1. Isolation of mononuclear cells from whole blood using Lymphocyte Separation Medium (LSM).
Examples of LSM applications include the following isolations:
- Mononuclear cell isolation from human peripheral blood: Liu et al. isolated monocytes from the peripheral blood of axial spondyloarthritis patients and healthy controls to study differential tumor necrosis factor alpha-induced protein 3 (TNFAIP3) regulation in blood-derived macrophages.2 This study demonstrated that monocytes isolated with LSM retain their differentiation capabilities and can produce functional macrophage colony-stimulating factor–derived macrophages for use in immunological studies.
- Mononuclear cell isolation from rat spleen cells: LSM has also been used successfully to isolate lymphocytes from rodent species even though the lymphocytes from rodents have a slightly higher average density than those from humans. In a recent study, the paracrine effects of mesenchymal stem cell (MSC)-derived exosomes, after acute myocardial infarction, on angiogenesis and anti-inflammatory activity was examined.3 In this case, rat spleen lymphocytes were isolated using LSM to perform T-cell proliferation assays used to assess the angiogenic potency of MSC-derived exosomes.
- Isolation of parasites from feces or blood: LSM has been demonstrated to be effective in isolating parasites from peripheral blood samples. Lymphatic filariasis is a tropical, mosquito vector–borne disease caused by filarial nematodes, such as Brugia malayi. Microfilariae (Mf) or first-stage larvae are ingested by mosquitoes that can infect humans through mosquito bites. Schroeder et al. successfully utilized LSM to isolate the Mf from the blood of infected gerbils.4 The Mf were co-cultured with human umbilical vein endothelial cells to study the mechanism of Mf sequestration in the lungs of infected individuals.
Lymphocyte separation for in vitro diagnostics
LymphoSep lymphocyte separation medium is based on the highly optimized Bøyum formulation, which was originally designed for the in vitro isolation of lymphocytes from diluted whole blood. It has a density of 1.077 g/mL. LymphoSep has been validated for in vitro diagnostic (IVD) usage and designated by the U.S. Food and Drug Administration (FDA) as a Class I medical device that is, as a lymphocyte separation medium, exempt from the FDA’s premarket notification requirements (21CFR864.8500). Like LSM, LymphoSep provides high yield of lymphocyte cells with more than 96% cell viability in one-step centrifugation.
Defibrinated or heparinized blood specimens are first diluted with physiological saline or balanced salt solution in 1:1 proportion, layered over the separation medium, and centrifuged at a low speed about 400 g for 15–30 minutes. During centrifugation, blood constituents differentially migrate into several layers.
When LymphoSep is used as the separation medium, the layers that form are like those formed when LSM is used (Figure 1). They are, from top to bottom, as follows: blood plasma and other constituents; the so-called buffy coat (which contains cells such as lymphocytes and monocytes); separation medium; and a precipitate (in the form a pellet at the very bottom of a conical tube) that contains granulocytes and erythrocytes (red blood cells). To isolate PBMCs, the buffy coat is extracted, washed with salt-buffered solution, and then centrifuged, allowing the cells to be recovered with high yield in a small volume. The supernatant, which contains platelets, separation medium, and plasma, is removed, leaving a pellet of purified PBMCs. These cells can then be used in life sciences applications, such as flow cytometry, cell sorting, cell culture, and sequencing procedures.
Polymorphonuclear cell isolation
Oftentimes, researchers want to do more than isolate the mononuclear cell population. When it is necessary to separate both mononuclear and polymorphonuclear cells from blood, mono-poly resolving medium (M-PRM) may be used. It is specifically designed for optimal separation of mononuclear lymphocytes and polymorphonuclear leukocytes from contaminating erythrocytes in a one-step centrifugation. M-PRM is a solution composed of a polysaccharide (Ficoll 400) and a radiopaque contrast medium (Hypaque) in a specific ratio to yield a density of 1.114 ± 0.002 g/mL.
Undiluted, anticoagulant-treated blood is layered over M-PRM prior to centrifugation (Figure 2). Differential migration during centrifugation allows for the resolution of both mononuclear and polymorphonuclear leukocytes into two distinct bands that are
relatively free of erythrocytes.
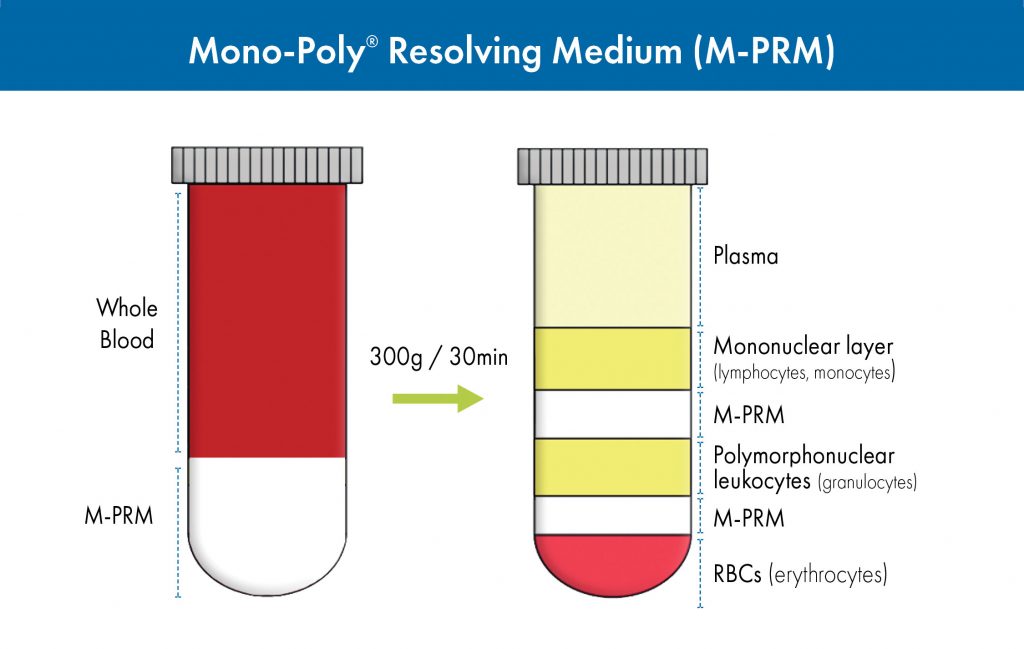
Figure 2. Isolation of both mononuclear and polynuclear cells from whole blood using Mono-Poly Resolving Medium (M-PRM).
Typical results from various laboratories have shown that separation of blood on M-PRM can achieve >90% leukocyte recovery with >99% viability as assessed by the trypan blue dye exclusion method. Generally, the top leukocyte band (fraction 1) contains 94–98% mononuclear cells (lymphocytes and macrophages) whereas the second (fraction 2) contains 96–99% polymorphonuclear leukocytes.
Both the mononuclear and polymorphonuclear leukocytes isolated by this system have been shown to retain functional properties. In particular, the polymorphonuclear cells fractionated using M-PRM have better functional capacities than those prepared by other methods. For example, M-PRM has been used for the isolation of neutrophils from blood for use in a wide variety of assays for immunological research. These phagocytic granulocytes, which act by ingesting and/or releasing enzymes that kill the microorganisms, are the first line of defense against infection. They are an essential part of the innate immune system.
The isolation of mononuclear and polymorphonuclear cells is usually the first and many times the most critical step in targeted cell sorting and separation procedures. It can provide a viable population of cells for subsequent steps, which typically employ magnetic bead-based systems or flow cytometers. Targeted separation is more efficient and cost effective if the starting material is a highly enriched fraction instead of whole blood. As cellular subpopulations of interest are extremely rare with limited sample size and volume, it is of upmost importance to obtain the highest yield of viable cells, without functional degradations, from your specimen of choice.
Shanghao Li, PhD (Shanghao.Li@mpbio.com), is global product manager, immunology and cell biology, MP Biomedicals, Santa Ana, CA. Micic Miodrag, PhD, ScD, is chief marketing officer, MP Biomedicals, Santa Ana, CA. He is also professor and department chair, engineering design technology, Cerritos College, Norwalk, CA. He was formerly vice president, R&D, at MP Biomedicals.
References
- Bøyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Invest. 1968; 21 (Suppl. 97): 77–89.
- Liu Y, et al. Genetic and functional associations with decreased anti-inflammatorytumor necrosis factor alpha induced protein 3 in macrophages from subjects with axial spondyloarthritis. Front. Immunol. 2017; 8: 860.
- Teng X, et al. Mesenchymal stem cell-derived exosomes improve the
microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell. Physiol. Biochem. 2015; 37: 2415–2424. - Schroeder J-H, et al. Brugia malayi microfilariae adhere to human vascular
endothelial cells in a C3-dependent manner. PLoS Negl. Trop. Dis. 2017; 11: e0005592.
Learn more about Lymphocyte Separation Medium (LSM™), LymphoSep™, and Mono-Poly™ Resolving Medium.
>> View article