
Antikörper (auch: Immunglobuline) werden im Zuge der adaptiven Immunantwort von B-Lymphozyten produziert. Nach ihrer Sekretion binden sie mit extrem hoher Spezifität an jenes Antigen, das ihre Produktion stimuliert hat. Auf diese Weise werden Pathogene für die Zerstörung markiert oder die Bindung von Viren an Wirtszellrezeptoren verhindert. Ihre hochspezifische Bindungskapazität macht Antikörper zu einem wertvollen Werkzeug für die Forschung. Seit Jahrzehnten finden sie breitflächig Anwendung in verschiedenen Immundetektionstechniken wie Western Blot, Immunzytochemie, Immunhistochemie (IHC), Durchflusszytometrie, ELISA und Lateral Flow Assays, um nur einige zu nennen.
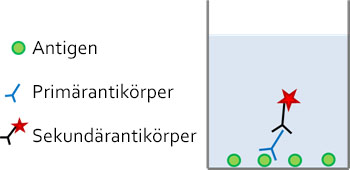 Ein typischer Immunassay wird zunächst mit einem Blocking-Reagenz inkubiert um unspezifische Bindungen zu minimieren. Anschließend wird der primäre Antikörper hinzugefügt, der spezifisch an das Ziel-Antigen bindet. Nach dem Auswaschen etwaiger ungebundener Antikörper, wird ein Sekundärantikörper zur Detektion verwendet. Sekundärantikörper sind meist Anti-Spezies-Antikörper gegen den Wirt, in welchem der Primärantikörper generiert wurde, und üblicherweise mit einem Farbstoff oder Enzym gekoppelt, um die Anwesenheit des detektierten Antigens in der Probe sichtbar zu machen.
Ein typischer Immunassay wird zunächst mit einem Blocking-Reagenz inkubiert um unspezifische Bindungen zu minimieren. Anschließend wird der primäre Antikörper hinzugefügt, der spezifisch an das Ziel-Antigen bindet. Nach dem Auswaschen etwaiger ungebundener Antikörper, wird ein Sekundärantikörper zur Detektion verwendet. Sekundärantikörper sind meist Anti-Spezies-Antikörper gegen den Wirt, in welchem der Primärantikörper generiert wurde, und üblicherweise mit einem Farbstoff oder Enzym gekoppelt, um die Anwesenheit des detektierten Antigens in der Probe sichtbar zu machen.
Abb: Immundetektion in einem kompetitiven ELISA-Assay. Das Antigen ist auf der Oberfläche einer Mikrotiterplatte fixiert. Seine Anwesenheit wird durch die Bindung eines Antigen-spezifischen Primärantikörpers und eines markierten speziesspezifischen Sekundärantikörpers nachgewiesen.
Ein Primärantikörper für die Immundetektion sollte einige Voraussetzungen erfüllen. Essentiell ist die Spezifität des Antikörpers, da unspezifische Bindungen zu einer Missinterpretation der Ergebnisse führen können. Daneben spielt seine Sensitivität eine Rolle, insbesondere, wenn die Testprobe nur eine geringe Menge des Antigens enthält. Die Spezifität eines Antikörpers sollte stets durch geeignete Positiv- und Negativkontrollen bestätigt werden. Als Positivkontrolle eignet sich beispielsweise ein Western Blot mit rekombinantem Protein oder Lysaten aus Zelllinien, die das Ziel-Antigen in hohem Maße exprimieren. Als Negativkontrolle können mikroskopische Aufnahmen von gefärbten Zellen dienen, die zuvor mit siRNA behandelt wurden. Unsere Antikörper-Hersteller stellen entsprechende Daten auf Anfrage zur Verfügung. Einige unserer Partnerfirmen validieren ihre Antikörper darüber hinaus besonders extensiv, mittels knockout/knockdown des Zielgens, durch verschiedene Techniken und mit unterschiedlichen Gewebetypen, um besonders hohen Qualitätsstandards zu entsprechen. Wir beraten Sie dazu gerne.
Auch ob ein polyklonaler oder ein monoklonaler Antikörper für einen Assay geeigneter ist, will durchdacht sein. Monoklonale Antikörper erkennen ein einzelnes Epitop eines Antigens und werden aufgrund ihrer definierten Spezifität und exakten Reproduzierbarkeit oft bevorzugt. Polyklonale Antikörper enthalten eine Antikörper-Population und erkennen unterschiedliche Epitope eines Antigens. Sie liefern eine gute Signalamplifikation und hohe Sensitivität zu dem Preis einer unter Umständen hohen Variabilität.
BIOZOL bietet eine extensive Auswahl an hochqualitativen primären und sekundären Antikörpern, die mit unserer Filterfunktion einfach durchsucht werden können, auf Basis von Speziesreaktivität, Anwendung, Klonalität, Wirtspezies, Isotyp und Hersteller. Sollten Sie nicht selbst fündig werden, helfen wir Ihnen gerne persönlich weiter: stellen Sie einfach eine Produktsuchanfrage über unser Formular und wir finden eine individuelle Lösung für Sie. Speziellen Wünsche können wir durch eine kundenspezifische Antikörpersynthese gerecht werden.
Erfahren Sie mehr aus unseren Broschüren, die ausgewählte Hersteller und Produkte zeigen:




 Ein typischer Immunassay wird zunächst mit einem Blocking-Reagenz inkubiert um unspezifische Bindungen zu minimieren. Anschließend wird der primäre Antikörper hinzugefügt, der spezifisch an das Ziel-Antigen bindet. Nach dem Auswaschen etwaiger ungebundener Antikörper, wird ein Sekundärantikörper zur Detektion verwendet. Sekundärantikörper sind meist Anti-Spezies-Antikörper gegen den Wirt, in welchem der Primärantikörper generiert wurde, und üblicherweise mit einem Farbstoff oder Enzym gekoppelt, um die Anwesenheit des detektierten Antigens in der Probe sichtbar zu machen.
Ein typischer Immunassay wird zunächst mit einem Blocking-Reagenz inkubiert um unspezifische Bindungen zu minimieren. Anschließend wird der primäre Antikörper hinzugefügt, der spezifisch an das Ziel-Antigen bindet. Nach dem Auswaschen etwaiger ungebundener Antikörper, wird ein Sekundärantikörper zur Detektion verwendet. Sekundärantikörper sind meist Anti-Spezies-Antikörper gegen den Wirt, in welchem der Primärantikörper generiert wurde, und üblicherweise mit einem Farbstoff oder Enzym gekoppelt, um die Anwesenheit des detektierten Antigens in der Probe sichtbar zu machen.