CE-IVD Mark
We are proud to announce the availability of quanty COVID-19 kit CE-IVD: "The Italian kit to fight the Italian outbreak!" The evaluation was performed in collaboration with Sacco Hospital University of Milan and University of Milan- Department of Biomedical Sciences for Health - Reference Laboratory for Respiratory Infections Surveillance. quanty COVID-19 allows the detection and quantification of the COVID-19 genome using Multiplex PCR, according to the CDC indications. Product development was made possible thanks to the hard work of the Clonit R&D Team, which made available a kit with such great clinical value in such a short time.
INTRODUCTION AND PURPOSE OF USE
The quanty COVID-19 system is a quantitative test that allows the quantification, by means of Real Time PCR, of the N region (nucleocapsid phosphoprotein) of novel Coronavirus (COVID-19).
The Procedure allows the detection of the RNA target by means a retro-amplification reaction in a microplate.
The analysis of the results is made using a Real Time PCR analyzer instrument (thermal cycler integrated with a system for fluorescence detection).
3 targets of the N* region:
N*1: Real Time Detection and Quantification
N*2: Real Time Detection
N*3: Real Time Detection
3 Positive controls for each region
4 standards for the quantification
2 Master Mixes:
- Mmix 1 for detection of N1 and N2
- Mmix 2 for detection of N3 and IC
48 Tests:
- 48 test for the detection of N1 and N2
- 48 test for the detection of N3 an IC
* (nucleocapsid phosphoprotein)

MATERIALS AND STRUMENTATION REQUIRED BUT NOT SUPPLIED
Disposable latex powder-free gloves or similar material;
Bench microcentrifuge;
Micropipettes and Sterile tips with aerosol filter;
Vortex;
Sterile plastic materials (microplate and optical adhesive cover);
Heat block (only for manual extraction)
Reagents
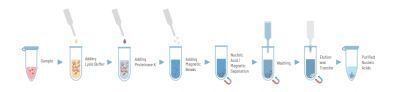
The quanty COVID-19 kit was developed and validated to be used with the following extraction method:
SAMPLES AND STORAGE
The quanty COVID-19 kit must be used with extracted RNA from biological samples: Nasopharyngeal/oropharyngeal swabs, sputum and serum. Collected samples must be shipped and stored at +2 - +8°C and used within 3 days from
PRECAUTIONS USE
This kit is for in vitro diagnostic (IVD), for professional use only and not for in vivo use.
After reconstitution, the amplification master mix must be used in one time. Repeat thawing and freezing of reagents (more than twice) should be avoided, as this might affect the performance of the assay. The reagents should be frozen in aliquots, if they are to be used intermittently. At all times follow Good Laboratory Practice (GLP) guidelines.
Wear protective clothing such as laboratory coats and disposable gloves while assaying samples.
Avoid any contact between hands and eyes or nose during specimen collection and testing.
Handle and dispose all used materials into appropriate bio-hazard waste containers. It should be discarded according to local law.
Keep separated the extraction and the reagents preparation.
Never pipette solutions by mouth.
Avoid the air bubbles during the master mix dispensing. Eliminate them before starting amplification.
Wash hands carefully after handling samples and reagents.
Do not mix reagents from different lots.
It is not infectious and hazardous for the health (see Material Safety data Sheet – MSDS).
Do not eat, drink or smoke in the area where specimens and kit reagents are handled.
Read carefully the instructions notice before using this test.
Do not use beyond the expiration date which appears on the package label.
Do not use a test from a damaged protective wrapper.
LIMIT OF THE METHOD
The extreme sensitivity of gene amplification may cause false positives due to cross-contamination between samples and controls. Therefore, you should:
- physically separate all the products and reagents used for amplification reactions from those used for other reactions, as well as from post-amplification products;
- use tips with filters to prevent cross-contamination between samples;
- use disposable gloves and change them frequently;
- carefully open test tubes to prevent aerosol formation;
- close every test tube before opening another one.
The proper functioning of the retro-amplification mix depends on the correct collection, correct transportation, correct storage and correct preparation of a biological sample.
As with any diagnostic device, the results obtained with this product must be interpreted taking in consideration all the clinical data and other laboratory tests done on the patient.
A negative result obtained with this product suggests that the RNA of COVID-19 was not detected in RNA extracted from the sample, but it may also contain COVID-19-RNA at a lower titre than the detection limit for the product (detection limit for the product, see paragraph on Performance Characteristics); in this case the result would be a false negative.
As with any diagnostic device, with this product there is a residual risk of obtaining invalid, false positives or false negatives results.
STORAGE AND STABILITY
Store the product quanty COVID-19 at –20°C.
The quanty COVID-19 kit is shipped on dry ice. The kit components should be frozen.
If one or more components are not frozen upon receipt or if the tubes have been compromised during transport, contact Clonit srl for assistance.
An intact and well stored product has a stability of 12 months from the date of production. Do not use beyond the expiration date which appears on the package label.
Repeat thawing and freezing of reagents (more than twice) should be avoided, as this might affect the performance of the assay. The reagents should be frozen in aliquots, if they are to be used intermittently. |