Targeting RNA Editing
SignalChem’s ADAR Products
The ADAR (Adenosine Deaminase Acting on RNA) family comprises enzymes that catalyze the deamination of adenosine (A) to inosine (I) within double-stranded RNA, a process known as A-to-I RNA editing, which affects RNA stability, splicing, and translation. ADAR1 is also associated with various diseases such as type I interferonopathies, cancer, and viral infections, making it a crucial drug target. Three members, ADAR1 (p150 and p110), ADAR2, and ADAR3, have been identified. ADAR1 and ADAR2 possess catalytic activity, while ADAR3 likely serves a regulatory function. Structurally, ADAR proteins contain double-stranded RNA-binding domains and a deaminase domain that confer substrate specificity.
SignalChem Biotech, now part of Sino Biological, has launched novel recombinant ADAR proteins: ADAR1 (p150), ADAR1 (p110), and ADAR2L (ADARB1). These products support research on RNA editing mechanisms, identification of RNA targets, and screening of potential modulators and inhibitors. In addition, SignalChem Biotech provides dedicated ADAR inhibitor screening services to accelerate drug discovery in this emerging field.
Featured ADAR Proteins
|
Human ADAR1 (p150) Protein (Art. N°: SCM-A601-310H) |
Human ADAR1 (p110) (Art. N°: SCM-A611-310GH) |
Human ADARB1 (ADAR2L) Protein (Art. N°: SCM-A602-310F) |
 |
 |
 |
| The purity of ADAR1 (p150) was determined to be >70% by densitometry. Calculated MW ~155 kDa. Observed MW ~155 kDa. | The purity of ADAR1 (p110) was determined to be >70% by densitometry. Calculated MW ~146 kDa. Observed MW ~138 kDa. | The purity of ADARB1 (ADAR2L) was determined to be >85% by densitometry. Calculated MW ~85 kDa. Observed MW ~82 kDa. |
 |
 |
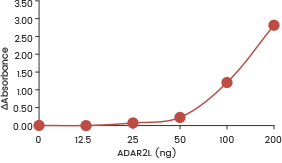 |
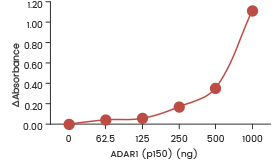
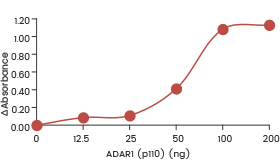
| Recombinant human ADAR1 (p150) (SCM-A601-310H) shows dose-dependent adenosine deaminase activity towards double-stranded RNA in an ELISA format assay. | Recombinant human ADAR1 (p110) (SCM-A611-310GH) showed dose-dependent adenosine deaminase activity towards double-stranded RNA in an ELISA format assay. | Recombinant human ADARB1 (ADAR2L) (SCM-A602-310F) shows dose-dependent adenosine deaminase activity towards double-stranded RNA in an ELISA format assay. |
Introduction to ADAR
ADAR Enzymes and RNA Editing
Compared with DNA editing, RNA editing can avoid the ethical and safety problems caused by direct modification of DNA. In addition, RNA editing is reversible and flexible, making it a more precise approach of gene therapy. A-to-I editing is one of the most prevalent modifications in RNA and refers to the deamination of adenosine (A) to inosine (I) on a double-stranded RNA (dsRNA) substrate. ADAR enzymes drive A-to-I editing, playing a central role in regulating RNA diversity, maintaining immune homeostasis, and supporting nervous system function. ADAR-based therapies are emerging as a precise approach in RNA editing, offering higher specificity compared to DNA editing methods like CRISPR-Cas9. In recent years, ADAR-mediated site-directed RNA editing has shown great potential in cancer therapy, as it can precisely modify individual nucleotides in oncogene transcripts to suppress tumor growth.
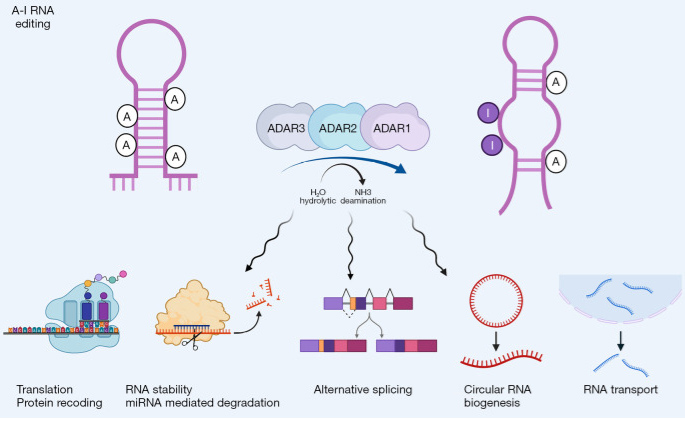
Physiological and Pathological Roles of ADAR
Physiologically, ADAR1 and ADAR2 regulate RNA splicing, stability, and translation, contributing to neural development, hematopoiesis, and innate immune homeostasis. ADAR1 edits endogenous double-stranded RNA to prevent inappropriate activation of MDA5-mediated interferon signaling, while ADAR2 modulates neurotransmission through editing of ion channel transcripts such as GRIA2. Pathologically, loss or mutation of ADAR1 leads to excessive interferon responses and autoimmune diseases like Aicardi-Goutières syndrome, whereas dysregulated RNA editing promotes tumor growth, metastasis, and resistance to therapy in various cancers. In viral infections, altered ADAR1 activity can either suppress antiviral immunity or facilitate viral persistence, highlighting its complex role in health and disease.
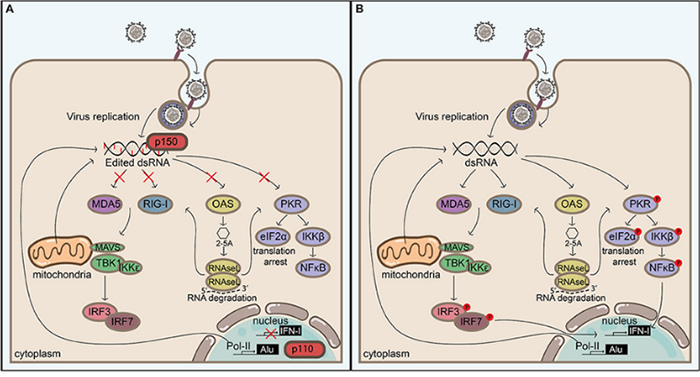
Advances in Drug Development Targeting ADAR
Recent clinical trials targeting ADAR and its inhibitors reflect an expanding effort to translate RNA editing into disease therapy. In cancer, small molecule inhibitors such as AVA-ADR-703 and CL-AD-100 are being tested in preclinical and planned clinical studies for malignancies and immunotherapy resistance, aiming to reverse ADAR1-driven immune evasion and drug resistance. On the other hand, ProQR Therapeutics is developing its proprietary Axiomer® platform, which harnesses endogenous ADAR enzymes to precisely edit RNA sequences, enabling the correction of disease-causing mutations in a range of genetic and liver-related disorders. While no FDA-approved ADAR inhibitor currently exists, ongoing first-in-human trials are evaluating safety, efficacy, and editing specificity, with future applications likely to extend to cancer, autoimmune, and viral diseases.
ADAR Inhibitors Screening Methods
ADAR inhibitors include small molecules targeting the Zα or deaminase domains, antisense oligonucleotides (ASO) that block substrate binding, and emerging PROTAC-based degraders designed to selectively eliminate ADAR1 protein. High-throughput screening methods for ADAR inhibitors commonly employ luminescent reporter assays in vitro, engineered cell lines or molecular docking approaches to rapidly identify compounds that suppress ADAR-mediated RNA editing activity. Based on the innovative platform, SignalChem Biotech provides a dedicated ADAR inhibitor screening service to accelerate drug discovery.
Contact us, if you want to learn more about the service.
27.01.2026

Discovery Research
Transfection solutions from Mirus Bio

ChIP-Exo-Seq
Validated Antibodies from Atlas Antibodies

Analytica 2026
Be our guest!
Metabolism Assays
Oxidative Stress, Glycolysis & Lipid Metabolism

New year, new Labort...
Up to 80% off labware

