COVID-19 diagnostics
qRT-PCR kits (CE IVD)
Due to the great demand, we took the PrimerDesign and Clonit quantitative real-time PCR kits (CE IVD) for diagnostic SARS-CoV-2 detection in stock for you at BIOZOL. These products are ready for shipment from Eching.
Clonit quanty COVID-19 new coronavirus (CE IVD): detection and quantification of the COVID-19 genome using Multiplex PCR
Clonit's assay detects and quantifies the N-region (nukleocapsid-phosphoprotein) of the novel Coronavirus (COVID-19) by Realtime PCR. The evaluation was performed in collaboration with Sacco Hospital University of Milan and University of Milan- Department of Biomedical Sciences for Health - Reference Laboratory for Respiratory Infections Surveillance. The RT-PCR quantitative COVID-19 Assay (CE-IVD) allows the detection and quantification of the COVID-19 genome using Multiplex PCR, according to the CDC indications.
>> quanty COVID-19 new coronavirus CE-IVD (CLO-RT-25)
Ingenetix ViroReal® Kit SARS-CoV-2 & SARS (CE IVD)
ViroReal® Kit SARS-CoV-2 & SARS is an in vitro diagnostic test for the detection of RNA for the N gene of SARS-CoV-2 and SARS-CoV by one-step reverse transcription real-time polymerase chain reaction (RT-PCR). The kit allows the rapid and sensitive detection of RNA of SARS-CoV-2 and SARS-CoV in samples from the respiratory tract.
>> ViroReal® Kit SARS-CoV-2 & SARS (ING-DHUV02313)

Logix Smart™ Coronavirus 2019 (COVID-19) Test Kit
The Logix Smart™ Coronavirus Disease 2019 (COVID-19) Test kit is an in vitro diagnostic test that uses Co-Diagnostics' patented CoPrimer™ technology for the qualitative detection of the RNA from SARS-CoV-2 coronavirus (COVID-19). The test operates using a single step real-time reverse transcriptase polymerase chain reaction (RT-PCR) process in lower respiratory tract fluids (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), and upper respiratory tract fluids (e.g. nasopharyngeal and oropharyngeal swabs) from patients who meet the clinical criteria (e.g. signs and symptoms) for coronavirus disease 2019 (COVID-19).
>> Logix Smart™ COVID-19 Test Kit (COD-COVID-K-001)

SARS-CoV-2 Triplex PCR kit (Form F) - CE IVD
The SARS-CoV-2 Triplex PCR kit is one-step reverse transcription real-time PCR multiplex test for detection of SARS-CoV-2 nucleic acids from nasopharyngeal swabs, oropharyngeal swabs and saliva samples from individuals suspected of COVID-19. The test simultaneously detects three targets - coding regions of the virus genome, each on separate detection channel.
>> SARS-CoV-2 Triplex PCR kit (Form F) - CE IVD (ATB-89-03)
Primerdesign qRT-PCR Kit (CE IVD) for clinical diagnosis of COVID-19
RNA extracted from patient samples can be analysed using the genesig Coronavirus (COVID-19) CE IVD test. The detection profile of the kit displays zero cross reactivity with other related viruses and 100% homology with all published SARS-CoV-2 sequences. This COVID-19 CE IVD kit with lyophilised reagents is available via BIOZOL with NO cold chain shipping. This is the ultimate solution to sensitive, rapid and cost-effective clinical diagnosis of COVID-19.
>> genesig® Real-Time PCR Coronavirus (COVID-2019) (PRD-Z-Path-COVID-19-CE)
.jpg)
Vitassay qPCR SARS-CoV-2 CE IVD
Vitassay qPCR SARS-CoV-2 allows the qualitative detection of 2019 Novel Coronavirus (SARS-CoV-2) by real-time RT-PCR in clinical samples. The product is intended for use in the diagnosis of SARSCoV-2 infections alongside clinical data of the patient and other laboratory tests outcomes. The ready-to-use test contains in each well all the necessary reagents for real-time PCR assay in a stabilized format. In addition, an internal control allows the detection of a possible reaction inhibition.
>> Vitassay qPCR SARS-CoV-2 CE IVD (Art.-Nr. BZL-7091046)

Quick-SARS-CoV-2 rRT-PCR Kit
The Quick SARS-CoV-2 rRT-PCR Kit is a real-time reverse transcription PCR (rRT-PCR) test for the qualitative detection of RNA from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which is responsible for the coronavirus disease (COVID-19). It targets three different regions of the viral nucleocapsid (N) gene and a host-specific target region (human RNase P gene) to assess sample quality. The kit also includes CV Positive Control that enable assay performance monitoring, and a No-Template Control to confirm the absence of contamination in the reagents.
>> Quick-SARS-CoV-2 rRT-PCR Kit (Art.-Nr. ZYM-R3011)
05.07.2021
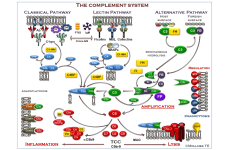
Complement System
WIESLAB® Functional Assays from Svar
Serum-free Organoid...
Afamin/Wnt3a Complex
Uncoated ELISA Kits
The cost-saving choice

Microplastics
Reference Materials from LGC Standards
CUT&Tag-IT
Genome-wide analysis of histone marks



